Background
Under the Toxic Substances Control Act (TSCA), companies are required to submit new substance notifications—including Premanufacture Notices (PMNs) and Low Volume Exemptions (LVEs)—before introducing new chemical substances into U.S. commerce. However, review times for these essential filings have long been unusually prolonged.
Multiple factors contribute to these delays. The U.S. Environmental Protection Agency (EPA) has faced persistent challenges such as insufficient funding, shortages of technical staff, and a large backlog of legacy projects. These systemic issues have hindered the EPA’s ability to complete reviews within TSCA’s legally mandated timeframes. Although the statute prescribes a 90-day review period for PMNs and 30 days for simplified exemptions like LVEs, on-time completion remains rare. This creates significant uncertainty for companies pursuing innovation and attempting to launch new chemical products, leading to serious delays in project timelines.
Companies can estimate actual review durations by checking the EPA's public database for the receipt and effective dates of notification. The agency generally follows a “first in, first reviewed” principle.
Figure 1: Example of EPA public disclosure for TSCA full-PMN exemption (LVE/LoREX/TME)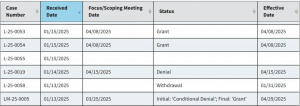 Figure 2: Example of EPA public disclosure for PMNs
Figure 2: Example of EPA public disclosure for PMNs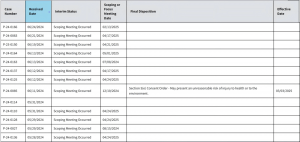
Latest Statistics Reveal Persistent Delays
According to the latest publicly available data: ● Between January 1 and May 23, 2025, the EPA received 87 full-PMN exemption (LVE/LoREX/TME). Of these, 67 have yet to receive a final decision, and none of the applications submitted since March 13 have entered the review process. ● From May 23, 2024, to May 23, 2025, the EPA received 142 PMNs/SNUNs, 127 of which remain undecided. These delays are due to various reasons, such as the EPA prioritizing older backlog cases, applicants needing time to compile information, the EPA re-reviewing amended submissions, or simply inefficient internal processes. Regardless of the cause, the net result for businesses is the same: without a decision, commercial activities cannot begin, severely disrupting market strategies.
U.S. vs. Canada: Significant Differences in New Substance Review Models
Unlike the Canadian Environmental Protection Act (CEPA), which allows commercial activity to proceed once the review window expires—even if the agency hasn't completed its review—TSCA does not permit silent approval.
In the U.S., companies must wait for an official EPA decision before manufacturing or importing new substances. Early commercial activity without EPA approval is illegal and may result in hefty civil fines and enforcement actions.
For example, General Motors Company and Ultium Cells LLC were fined between $290,000 and $650,000 for failing to file a PMN before importing a new substance.
Figure 3: EPA Civil Enforcement Action – General Motors Company and Ultium Cells LLC Figure 4: EPA Civil Enforcement Action – Shepherd Chemical Company
Figure 4: EPA Civil Enforcement Action – Shepherd Chemical Company
Ongoing Efforts vs. Persistent Backlog
To reduce review timelines, the EPA has undertaken several initiatives, such as hosting webinars to clarify application requirements, hiring third-party assessors, requesting increased federal funding, and raising application fees. Yet despite these efforts, review durations have not significantly improved and still fall well short of the statutory 30-day or 90-day limits. The EPA receives approximately 500 new substance notifications annually. As of May 1, 2025, the agency still had 421 pending PMN/SNUN/MCAN filings and 137 simplified applications (LVE/LoREX/TME), underscoring the severity of the backlog.
EPA Reorganization: A Turning Point?
To address this mounting challenge, the EPA has initiated a major internal reorganization.
In a May 3 interview with Newsweek, EPA Administrator Lee Michael Zeldin criticized the previous administration for high building vacancy rates, low staff attendance, and soaring expenses. He noted that hundreds of new chemical approvals and over 12,000 pesticide reviews were overdue, well beyond statutory timelines.
Starting in May, the EPA began redistributing staff. Of the approximately 1,500 employees in the Office of Research and Development (ORD), about 400 will be reassigned, while others may face layoffs. Staff from the Safer Choice program will be reassigned to the TSCA new chemicals division, and the Energy Star program may be canceled altogether. While these changes may negatively impact programs like Safer Choice and Energy Star, they signal positive movement for the Office of Chemical Safety and Pollution Prevention (OCSPP), which oversees pesticide and new chemical registrations. Rather than losing staff, OCSPP will gain about 130 scientists, bioinformaticians, and IT specialists, all redirected toward TSCA new chemical evaluations.
While these changes may negatively impact programs like Safer Choice and Energy Star, they signal positive movement for the Office of Chemical Safety and Pollution Prevention (OCSPP), which oversees pesticide and new chemical registrations. Rather than losing staff, OCSPP will gain about 130 scientists, bioinformaticians, and IT specialists, all redirected toward TSCA new chemical evaluations.
Analysts caution that even with 130 new experts, it could take up to 18 months to see tangible improvements. New staff will require training and time to adapt, and the backlog may persist unless EPA also streamlines internal workflows and improves communication.
REACH24H Recommendations
Given that actual TSCA review times significantly exceed statutory limits, REACH24H recommends that companies:- Allocate ample buffer time for EPA review in their product launch schedules.
- Submit complete, high-quality documentation to minimize the risk of delays due to supplemental information requests.