Overview
The new overarching rule on cosmetics in China - Cosmetic Supervision and Administration Regulation (CSAR) was released by China State Council on June 29, 2020. With CSAR that came into effect on January 1, 2021, the cosmetics industry in China is entering a new era. Cosmetic products now must be registered, notified/filed with NMPA before being imported and sold legally in China.
Definition of Cosmetic Products
| Aspects | Cosmetics | Not Cosmetics |
| Usage | Spreading, spraying, or other similar ways such as rubbing | Injection, digestion, or oral administration |
| Applied body parts | External parts of the human body, such as skin, hair, nails, and lips | Oral mucosa or internal genitalia |
Functions and purpose of use | For cleansing, protecting, beautifying, or improving the appearance | Disease prevention or treatment |
| N.B.: • Toothpaste is not defined as a cosmetic but shall be regulated with reference to general cosmetics regulations. • CSAR does not apply to soaps, excluding those claimed to have special cosmetic efficacy (e.g., whitening). | ||
Classification of Cosmetic Products
Special Cosmetics vs. General Cosmetics
| Special Cosmetic Products | General Cosmetic Products |
i. Hair dyes ii. Hair perming products iii. Freckle-removing (whitening) products iv. Anti-hair loss products v. Sunscreens vi. Cosmetics with new efficacy | Other cosmetics, except for special cosmetics. |
| N.B.: •The five previously classified as special-use cosmetics - hair growth, depilating, breast beauty, slimming, and deodorant products registered before January 1, 2021, can continue to be manufactured, imported, and sold in China during the 5-year transition period. | |
Domestic Cosmetics vs. Imported Cosmetics
| Domestic cosmetics | Imported cosmetics |
The last process of contacting cosmetic contents is completed in the Chinese Mainland. | The last process of contacting cosmetics contents is completed out of Chinese Mainland. |
| N.B.: If the product’s last process of contacting cosmetic contents is completed in Hong Kong S.A.R, Macao S.A.R, or Chinese Taipei, it shall be managed with reference to the requirements of imported cosmetics. | |
NMPA Registration or Notification/Filing of Cosmetic Products
| Classification | Special cosmetics | General cosmetics |
| Pre-Market Obligation | Registration | Notification/Filing |
| Involved Entities | Registrant (domestic) Responsible Person (imported) | Notifier (domestic) Responsible Person (imported) |
| Competent Authority | NMPA | NMPA or local provincial MPA |
| Certificate | Registration license (Valid for five years) | E-certificate (Permanent) |
| Post-Market Obligation | Registrational renewal | Annual report submission |
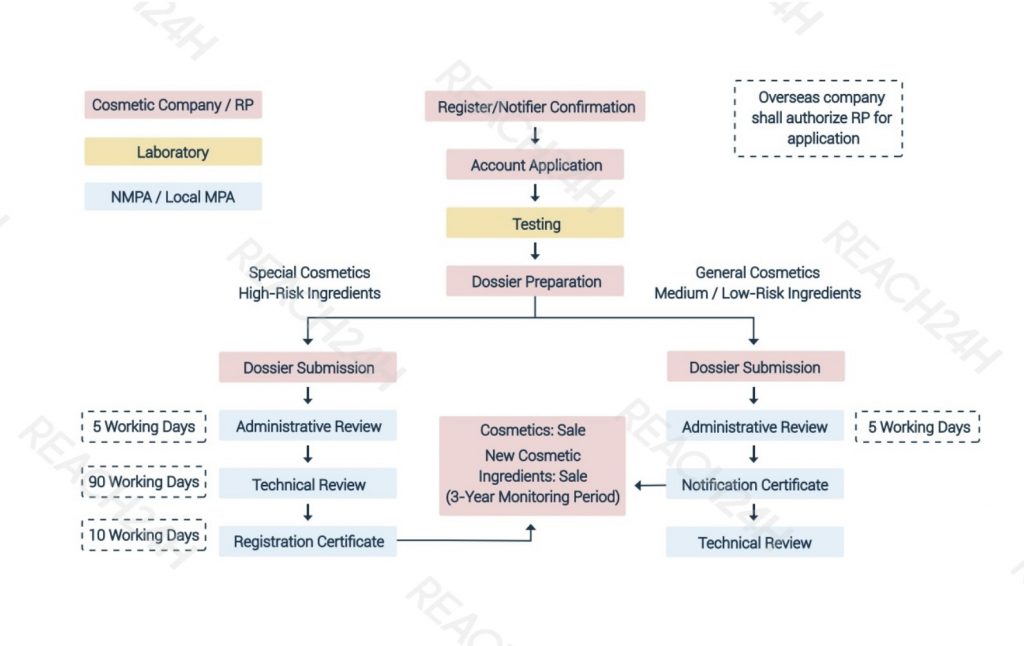
Steps for Imported Cosmetic Registration or Notification/Filing
Competent Authorities in China
National Medical Product Administration (NMPA)
It is responsible for the registration of imported/domestic special cosmetics, the notification of imported general cosmetics, and the registration and notification of new cosmetic ingredients.
State Administration for Market Regulation (SAMR)
Its main responsibilities include market management and quality and safety supervision.
General Administration of Customs (GAC)
It is in charge of the inspection of imported and exported goods and quarantine of cosmetics.
NMPA Registration and Notification/Filing Dossiers
Account Application and User Permission Opening
The registrant and notifier information form and resume of the person in charge of quality and safety
Quality management system overview of registrants and notifiers
Adverse reaction monitoring and evaluation system overview form of registrants and notifiers
Domestic responsible person information form (applicable to overseas registrants and notifiers)
The original authorization letter of the domestic responsible person and its original notarial certificate
Registration and Notification/Filing Application
Cosmetics registration and notification information form and related documents (classification code)
Product name information
Product formula (ingredient submission code)
Product executive standards
Product label sample manuscript
Product testing report
Product safety assessment documents
Other supporting documents
N.B:
Imported cosmetics are required to submit certifications relating to manufacturing quality control of the overseas manufacturers.
For products specially produced for the China market without the supporting documents, the applicant shall submit relevant research and test data for Chinese consumers.
Our Services
Cosmetics Regulatory and Technical Advisory
Cosmetics Registration/Notification
China Responsible Person
Cosmetic Product Safety Report (CPSR)
Why Choose Us?
Owns a leading online information platform that provides timely insights into regulatory and market intelligence
Industry-leading expertise in cosmetics compliance consulting services
Served over 1000+ cosmetics companies worldwide
Established cooperative relationships with governmental authorities, industry associations, and laboratories
A professional, global, multilingual, and multicultural team with the capability to deliver services with high efficiency through a systematic approach
For more information and inquiries on China's cosmetics registration, please feel free to contact us at customer@reach24h.com.