Since June 2021, plant-based ingredients have become a major trend in China’s cosmetic industry, accounting for a substantial share of new ingredient registrations. Encouraged by national and local policies, companies are using regional botanical resources and technological expertise to develop unique plant-based cosmetic ingredients. The recently published Work Plan for Stabilizing Growth in the Light Industry (2023–2024)reinforces the importance of developing innovative raw materials from plants.
However, registering plant-based cosmetic ingredients in China presents unique challenges compared to synthetic alternatives. The natural complexity and variability of plant extracts require careful consideration of the regulatory requirements. In this article, REACH24H outlines the key requirements and challenges companies need to understand when registering new plant-based cosmetic ingredients in China.
Essential Information for Plant-Based Ingredient Registration
When registering a new plant-derived ingredient, companies must provide comprehensive data about its botanical origin, including:
- Common and Latin name (scientific classification, genus, and species)
- Source identification (authentication report from a qualified professional or institution)
- Plant parts used (eg, leaves, roots, stems, flowers)
- Geographical origin and cultivation method (wild-harvested or cultivated)
Standardized Naming of Plant-Based Ingredients
To ensure compliance, plant-based ingredients should be named according to the International Nomenclature of Cosmetic Ingredients (INCI) in Chinese Standards.
Directly Derived Plant Ingredients
For ingredients extracted directly from plants, the naming should follow the format:
[Chinese Name] + [Latin Name] + [Plant Part Used] + [Form of Use]Examples:
- 平卧菊三七(GYNURA PROCUMBENS)提取物 (National Cosmetic Ingredient Filing No. 20230013): According to the disclosed production process, this ingredient is extracted from the whole plant of Gynura procumbens.
- 大叶冬青(ILEX LATIFOLIA)叶提取物 (National Cosmetic Ingredient Filing No. 20230021): The name clearly specifies the plant’s Latin name (Ilex latifolia) and the part used (leaf).
Indirectly Derived Plant Ingredients
If the ingredient is obtained through plant tissue culture or fermentation, a more descriptive name should be used.Example:
- 冰叶日中花(MESEMBRYANTHEMUM CRYSTALLINUM)愈伤组织提取物 (National Cosmetic Ingredient Filing No. 20230017): This Ingredient is derived from plant tissue culture (callus) of Mesembryanthemum crystallinum.
Composition and Additives in Plant-Based Ingredients
Unlike synthetic chemicals, plant-based ingredients are complex natural mixtures. While purity levels cannot be defined as with single-compound chemicals, the key chemical constituents (such as polyphenols, flavonoids, polysaccharides, peptides, and saponins) must be specified.Additionally, plant extracts may be presented in powder or paste form. If processing requires auxiliary agents such as solvents, preservatives, or stabilizers, these must also be declared.
Safety Assessments: Addressing Pesticide Residue Risks
Due to agricultural practices, plant-based ingredients may contain pesticide residues, which necessitate safety assessments. The Chinese national standard GB/T 39665-2020, issued by the State Administration for Market Regulation in December 2020, provides a reference method for testing 55 prohibited pesticide residues in plant-derived cosmetic ingredients.
Toxicological Data Requirements for Plant-Based Ingredients
Unlike synthetic compounds, there are no exclusive toxicological data requirements for plant-derived ingredients. The required safety data will depend on the ingredient’s intended use, its biological activity, and the historical use in cosmetics or food.Toxicological testing requirements differ based on the different types of situations: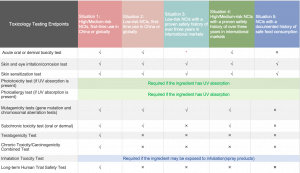
Alternative Testing Methods to Reduce Animal Testing
To align with global cruelty-free trends, China allows the use of alternative toxicology tests where applicable. However, most OECD-approved in vitro methods are designed for single-compound chemicals, which may not be suitable for complex plant extracts.For example, the Direct Peptide Reactivity Assay (DPRA) cannot be applied to plant-based materials due to their unknown composition and variability. When conducting alternative toxicology tests, companies must ensure methodological suitability for plant extracts.
Final Thoughts: Navigating Regulatory Challenges
With increasing regulatory support and market demand for plant-derived ingredients, understanding the registration and filing requirements is crucial for cosmetic brands and ingredient suppliers looking to enter or expand in China’s market.At REACH24H, we specialize in helping companies navigate regulatory challenges and register plant-based cosmetic ingredients through services that include:
- Ingredient authentication and naming compliance
- Regulatory filing strategies
- Toxicological risk assessment
- Pesticide residue testing
Need help registering a plant-based cosmetic ingredient in China? Contact REACH24H for expert support at customer@reach24h.com.





