Introduction
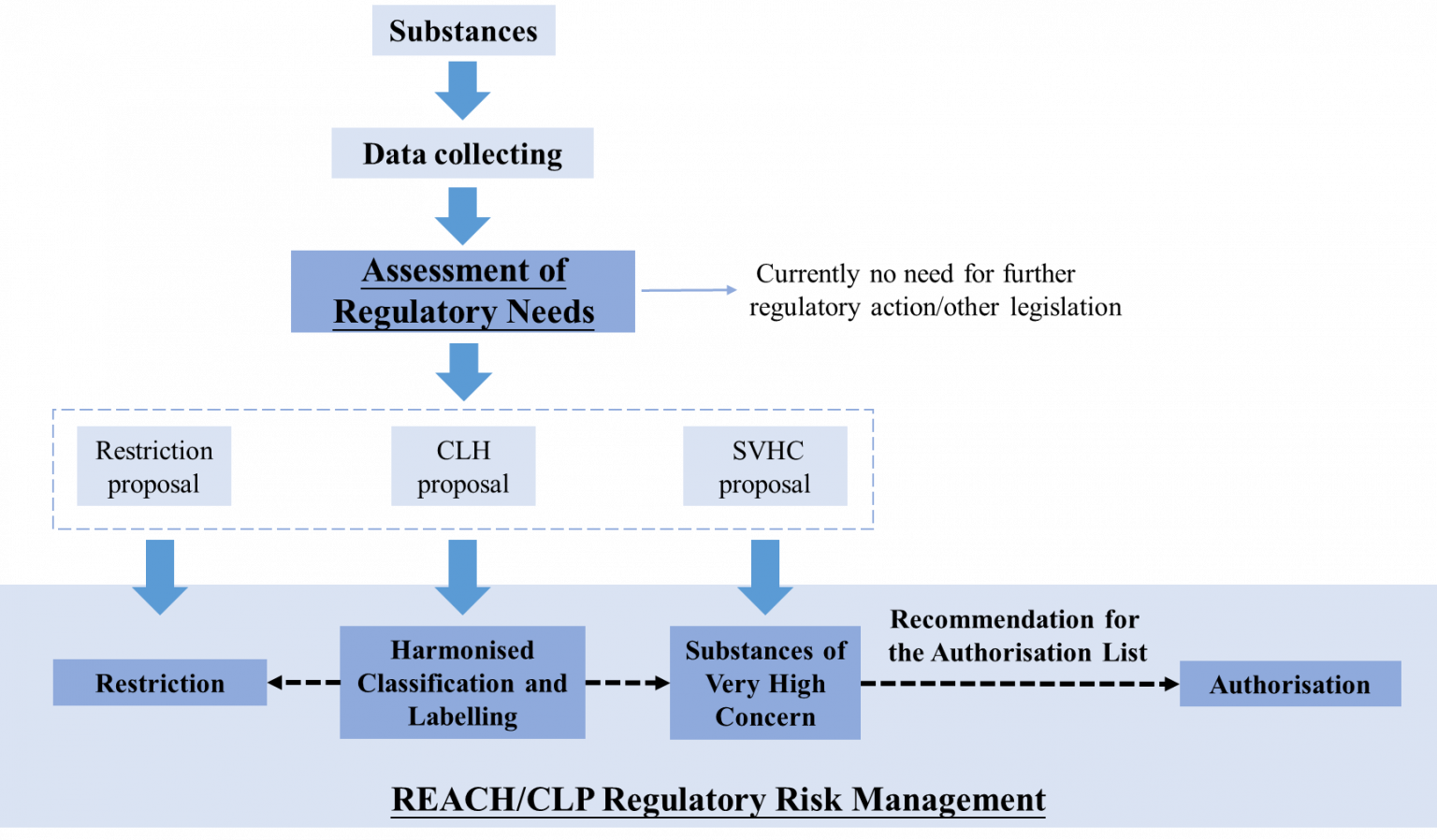
The EU REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation is widely recognized as one of the most stringent chemical management systems globally. Its core aim is to effectively control and ultimately eliminate hazardous substances, thereby minimizing the potential risks these substances pose to human health and the environment. Within this framework, the registration and evaluation of chemicals are merely the starting points of compliance. The key to achieving a high level of protection lies in its precise risk-based management mechanism, which primarily operates through four synergistic measures: Harmonized Classification and Labelling (CLH), Substances of Very High Concern (SVHC), Authorization, and Restriction.
Harmonized Classification and Labelling (CLH): The Unified Language of Risk Identification
Harmonized Classification and Labelling (CLH) is the cornerstone of the REACH risk management system, providing core data support for subsequent measures (SVHC identification, authorization, and restriction). Typically, the classification and labeling of substances/mixtures are carried out by enterprises themselves.
However, in specific circumstances, such as when a substance is identified as carcinogenic, mutagenic, or toxic for reproduction (CMR categories 1A/1B), a respiratory sensitizer, an endocrine disruptor (ED), a persistent, bioaccumulative and toxic (PBT) or very persistent and very bioaccumulative (vPvB) substance, or a persistent, mobile and toxic (PMT) or very persistent and very mobile (vPvM) substance, or when the EU level deems it necessary to harmonize classification, the European Commission will mandate its classification harmonization.
This harmonized classification will be included in Annex VI of the CLP Regulation and the C&L Inventory to ensure a consistent understanding of substance hazard properties across the entire EU, thereby guaranteeing the effectiveness of risk management measures.
For businesses, the core obligation is to apply these harmonized classification labels and accurately transmit compliant Safety Data Sheets (SDS) or extended Safety Data Sheets (eSDS) throughout the supply chain. This means businesses must continuously monitor updates to the C&L Inventory and promptly adjust product information.
Substances of Very High Concern (SVHC): Early Warning Signals for High-Risk Substances
Substances of very high concern (SVHC) are a critical risk management category in the REACH regulation, specifically referring to chemicals that pose serious and irreversible risks to human health or the environment. According to Article 57 of the REACH Regulation, the following categories of substances will be identified as SVHCs:
Carcinogenic, mutagenic or toxic for reproduction (CMR) substances: Specifically refers to substances classified as Category 1A or 1B.
Persistent, Bioaccumulative, and Toxic (PBT) substances: Refers to substances that are difficult to degrade in the environment, easily accumulate in organisms, and are toxic.
Very Persistent, Very Bioaccumulative (vPvB) substances: Substances that are even more difficult to degrade and have higher bioaccumulation than PBT substances.
Substances of equivalent concern: Even if they do not meet the above CMR, PBT, or vPvB criteria, but there is scientific evidence indicating that they may have serious effects on human health or the environment, such as endocrine disruptors.
The SVHC candidate list is a transition in the REACH regulation from hazard identification to stricter control; therefore, SVHC identification is only the first step towards inclusion in the authorization list, not the end of regulation. Its core value lies in mandating supply chain information transparency.
Information Communication in the Supply Chain:
If the SVHC content in a substance or mixture exceeds 0.1%, a REACH-compliant SDS must be passed down the supply chain.
If the SVHC content in an article exceeds 0.1%, safety instructions, including at least the name of the SVHC, must be provided to the downstream recipient. Consumers can also make similar requests, and the supplier should provide the relevant information free of charge within 45 days.
Notification Obligations:
When the SVHC content in an article exceeds 0.1% and the export volume exceeds 1 ton/year, EU producers, importers, or sole representatives must also submit an SVHC notification to ECHA.
When the SVHC content in an article merely exceeds 0.1%, EU producers and importers should also submit a SCIP notification to ECHA25.
The SVHC information, communication, and notification obligations compel businesses not only to understand the chemicals they directly use but also to deeply understand the chemical composition of the articles they purchase (such as parts and finished products). This series of measures substantially promotes the transparency of chemical information throughout the entire supply chain, enabling risks to be identified and managed from the source, thereby significantly reducing chemical risks across the market.
Authorization: A "Temporary Pass" for High-Risk Substances
Substances included in the SVHC list are regularly evaluated, and substances with higher hazard characteristics are further included in the scope of authorization control. The authorization mechanism is a core tool for managing high-risk substances under the REACH regulation, and its fundamental purpose is to ensure that the risks of these substances are appropriately controlled and to promote their gradual replacement by safer alternatives. Authorization can be understood as a "permit" system, where specific substances may not be placed on the EU market or used for specific purposes without explicit authorization from ECHA.
Key Milestones:
Latest Application Date (LAD): Enterprises must submit an authorization application before this date to continue use after the "Sunset Date".
Sunset Date: The final deadline for placing the substance on the market/use. After this date, unauthorized use is prohibited. Enterprises that submit applications before the LAD can continue to use the substance during the evaluation period 35.
Core Authorization Application Documents:
Chemical Safety Report (CSR): Details risk management measures and operating conditions.
Analysis of Alternatives (AoA): Evaluates whether safer, technically and economically feasible alternatives exist.
Socio-Economic Analysis (SEA): Weighs the benefits and risks of continued use and compares them with alternative solutions.
Substitution Plan: If a feasible alternative exists, a detailed phase-out timetable and plan must be submitted.
Authorization is not a permanent "pass," but a "conditional" permit. The intention of the authorization mechanism is not only to manage risks but also to serve as a transitional management tool that promotes phase-out and substitution. While ensuring controllable short-term risks, it provides strong economic and regulatory incentives for a long-term transition to safer chemicals.
Restriction: The Final Line of Defense for Risk Control
The restriction mechanism is the final line of defense in the REACH regulation's risk management system, used to manage substances that pose "unacceptable risks" to human health or the environment. When these risks cannot be adequately managed through other REACH procedures (such as registration, SVHC, authorization) or existing EU legislation, restriction measures are initiated.
The restriction conditions in Annex XVII are diverse, highly flexible, and targeted. They can range from complete prohibition to setting concentration limits, specific use restrictions, or even phased implementation dates. Unlike the "permit system" of authorization, restriction is the most direct "prohibition/restriction order". However, because it is a refined control measure for specific risk scenarios, it can minimize unnecessary economic impact while maximizing risk reduction.
Summary and Outlook
The EU REACH regulation, through the progressive layers of CLH, SVHC, authorization, and restriction, has built a sophisticated tiered risk management system. CLH and SVHC, as tools for hazard identification and communication, lay the foundation for information transparency and early risk warning. Authorization and restriction, on the other hand, serve as risk control measures, ensuring that the most appropriate and effective measures are taken for chemicals with different risk levels and natures. This tiered management system enables REACH to efficiently allocate regulatory resources, focusing the strictest regulatory measures on the highest-risk substances, while ensuring that all chemicals receive appropriate risk management and providing businesses with a clear compliance path and predictability.
For substances with lower hazard levels or controllable risks, information transparency through CLH is sufficient.
For highly hazardous substances not yet subject to authorization, the SVHC list provides early warning and information disclosure.
For highly hazardous and difficult-to-substitute substances, strict control is implemented through authorization.
For substances with unacceptable risks that cannot be managed by other means, direct prohibition or strict restriction is applied through restriction 57.
Although REACH has made significant strides in chemical management in theory and practice, laying a good foundation for global chemical management, striking a delicate balance between the noble goal of protecting human health and the environment and maintaining the economic competitiveness and innovation of EU industry remains a core challenge for its future reform.
Future REACH reform objectives will include simplifying the authorization process, introducing the concept of "essential uses," strengthening digitalization and enforcement, and other measures, aiming to continue strengthening health and environmental protection while reducing administrative burdens on businesses and improving efficiency and predictability.
For more information or service inquiries, please feel free to contact us at customer@reach24h.com!