Overview
Under AICIS, Industrial chemicals are those with an industrial use - this covers a broad range of chemicals used in inks, plastics, adhesives, paints, glues, solvents, cosmetics, soaps and many other products. Industrial chemicals include chemical elements, compounds, UVCBs, chemicals released by an article and naturally-occurring chemicals, etc, except for radioactive chemicals.
In addition, similar to chemical management regulations in other countries/regions, the AICIS regulatory system also includes exemptions for special substances or situations. If the industrial chemicals introduced by the enterprise are not within the exemption scope, the AICIS registration must be completed and relevant regulatory obligations must be fulfilled.
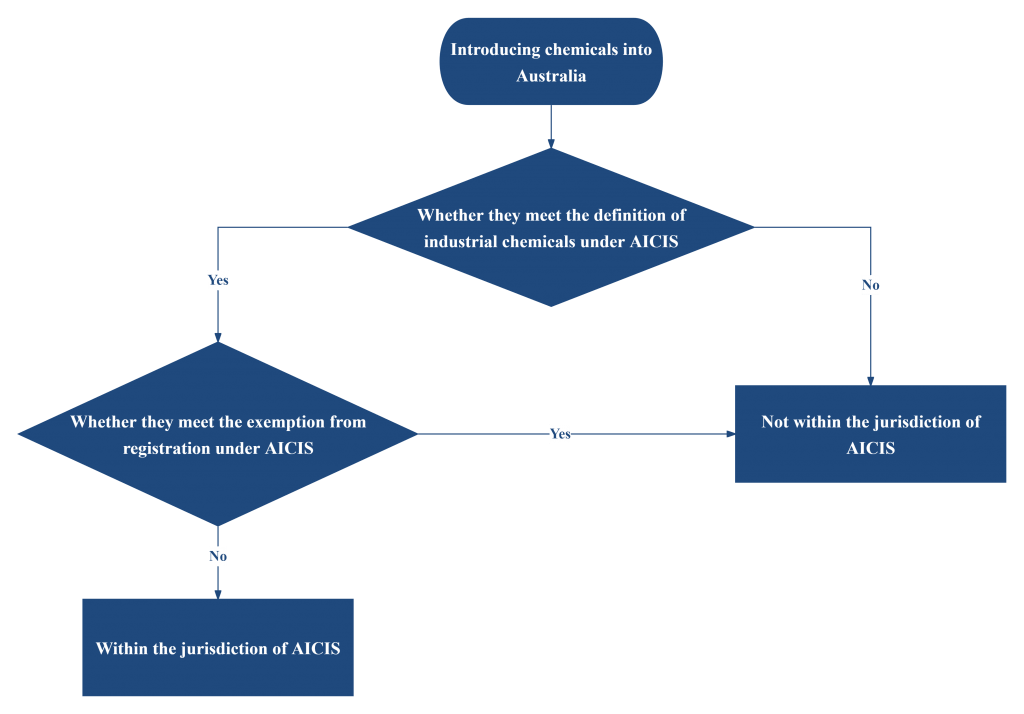
To determine whether the introduction of chemical substances is under the jurisdiction of AICIS, a two-dimensional verification is required: first, confirm whether it meets the definition of industrial chemicals, and second, check whether it is within the exemption scope.
Scroll down for more information from our expert team.
What is an Industrial Chemical?
AICIS defines industrial use by exclusion. This means that an industrial use is any use that isn’t:
Agricultural chemical product, veterinary chemical product as defined by the Agvet Code under APVMA (Australian Pesticides and Veterinary Medicines Authority) , and substance or mixture of substances prepared by a pharmacist or veterinary surgeon as defined by paragraph 5(4)(a) of the AgVet Code. Agricultural and veterinary use includes:
agriculture, including household gardening products
pesticides, including household products such as personal insect repellents, insect sprays and weed killers
pool sanitising chemicals such as sodium hypochlorite, sodium bromide and algaecides
veterinary medicine, pet food and livestock feed, etc.
Therapeutic good as defined by the Therapeutic Goods Act 1989 under TGA (Therapeutic Goods Administration). For example:
medical devices including sterilants and disinfectants and in vitro diagnostic devices (IVDs)
medicines including prescription, over-the-counter and complementary/alternative medicines
Primary sunscreen products(Conform to the AS/NZS 2604 "Sunscreen products - Evaluation and classification" standard under TGA)
Food as defined bythe Australia New Zealand Food Standards Code under FSANZ (Food Standards Australia New Zealand). For example:
Food for humans
Food additives
An important note about use is that a chemical can have multiple types of uses. For each use your chemical has, you need to follow the regulations for each responsible regulator.
What are the Exemption Situations for Registration under AICIS?
Chemicals introduced under the following circumstances are exempt under AICIS and usually do not require registration or introduction categorization:
Australian-made soap, meet all 4 criteria below:
The soap chemical must be made (manufactured) in Australia
The soap chemical must be made using a saponification process with a fat or oil and lye(either aqueous sodium hydroxide or aqueous potassium hydroxide)
The fat or oil you use to make the soap chemical must be on the Inventory
The total volume of every fat or oil you use to make the soap chemical must not be morethan 10 kg in an AICIS registration year (1 September to 31 August).
From the restrictions on the use of raw materials, this situation basically only applies to the situation of making a small amount of soap by individuals/families.
Selling products made from locally sourced ingredients, meet all 2 criteria below:
All the ingredients of this product are sourced locally from Australia
The product is obtained solely through physical blending of these ingredients
This is because the supplier or manufacturer of those ingredients that you bought from are already registered with AICIS.
Naturally occurring chemicals
This is legally defined as an unprocessed chemical occurring in a natural environment, or, achemical occurring in a natural environment that is extracted without chemical change by:
manual, mechanical or gravitational means; or
dissolution in water; or
flotation; or
a process of heating for the sole purpose of removing uncombined water
Ingredients that are labelled or marketed as ‘natural’, but their extraction and manufacturing processes are likely to involve chemical changes, such as fermentation, solvent extraction, etc., and therefore do not meet the definition of natural substances under AICIS.
Non-isolated intermediates, meet all 4 criteria below:
produced in the course of the manufacture of another industrial chemical
consumed in the course of the manufacture of the other industrial chemical
not intentionally removed from the equipment in which it is manufactured (other than by sampling)
not likely to be released into the environment during normal operations
Incidentally introduced chemicals
An incidentally introduced chemical is an industrial chemical that is introduced – either with or subsequent to the introduction of another industrial chemical – and has no commercial value separate from the other industrial chemical. For example:
the incomplete reaction of starting materials or reaction intermediates used in the manufacture of the other industrial chemical
an unintended constituent present in the starting materials used in the manufacture of the other industrial chemical
the exposure of the other industrial chemical to light, heat or other environmental conditions in the course of handling or storage
the occurrence of a chemical reaction during the manufacture or use of the other industrial chemical
Articles
An article must meet all 3 criteria below:
It is produced for use for a particular purpose, being a purpose that requires that the object have a particularshape, surface or design;
It is formed to that shape, surface or design during production;
It undergoes no change of chemical composition when used for that purpose except as an intrinsic aspect of thatuse.
Importantly, these objects are not articles:
objects that are a piece of matter whose purpose is determined to a lesser degree by the object’s shape, surface or design, compared to the object’s chemical composition. In other words, the object’s chemical composition ismore relevant to the function of the object than its shape, surface or design.For example, soap.
objects that are wholly fluid - this includes liquid, gas and plasma. For example, perfume.
| Examples of articles | Furniture such as tables and chairs Plastic and wooden toys Books, CDs and DVDs Clothing and shoes Whitegoods and kitchen appliances such as dishwashers, toasters, refrigerators Empty plastic or glass bottles Cookware, crockery and cutlery A closed cell battery |
| Examples that are not articles | Shampoo, conditioner, skin lotion soap perfume and other cosmetic products Cleaning products such as detergent, soap and bleach Fire extinguishers Wax crayons, ballpoint pens, permanent markers and whiteboard markers Glue sticks |
Chemicals unintentionally released from an article
Means the release of a chemical from an article, where the article was not designed to do this. Examples include:
chemicals leaching from a plastic chair during use or disposal
chemicals leaking from a sealed, closed cell battery
If the article has been designed to release chemicals, then the released chemicals need to be registered with AICIS and fulfill other regulatory obligations, such as the chemicals contained in the ink of ballpoint pens.
Transshipment chemicals, meet all 3 criteria below:
is introduced at a port or airport in Australia
remains subject to customs control under the Customs Act 1901 at all times before leaving Australia
leaves Australia within 25 working days beginning the day the industrial chemical is introduced
Chemicals introduced incidentally on an aircraft or ship, meet all 3 criteria below:
introduced incidentally to the carriage of passengers or the importation of other products on an aircraft or ship that leaves Australia within 25 working days
used to support the operation of the aircraft or ship
not freight
Chemicals introduced only for personal use and NOT in any way connected with a business activity
Therefore, to determine whether the introduction of chemicals in Australia is within the scope of AICIS supervision, a comprehensive judgment should be made based on the use of the substance and the introduction situation.
If the substance is determined to meet the definition of industrial chemicals by AICIS and is not exempt from registration, commercial registration must be completed and compliance obligations must be fulfilled in accordance with AICIS requirements.
REACH24H's Reminder
REACH24H reminds relevant enterprises to gain an in-depth understanding of the determination rules for the management scope of AICIS to avoid non-compliant introduction activities.
For more information or service inquiries, please feel free to contact us at customer@reach24h.com!