Introduction
In recent years, exosomes have emerged as a focal point in both academic research and the biomedical industry, thanks to their vast potential in medical diagnostics, treatment, and regenerative medicine. The United States, a strategic hub for exosome R&D and commercialization, continues to attract international enterprises eager to stake a claim in this rapidly evolving frontier.
Last week, Ruiou BaiPharm, a pharmaceutical-consulting-oriented subsidiary of REACH24H, assisted a China-based biopharmaceutical company in obtaining Type II Drug Master File (DMF) registration from the U.S. Food and Drug Administration (FDA) for their independently developed umbilical cord mesenchymal stem cell exosome subpopulations S1 (DMF No. 041763) and S2 (DMF No. 041762).

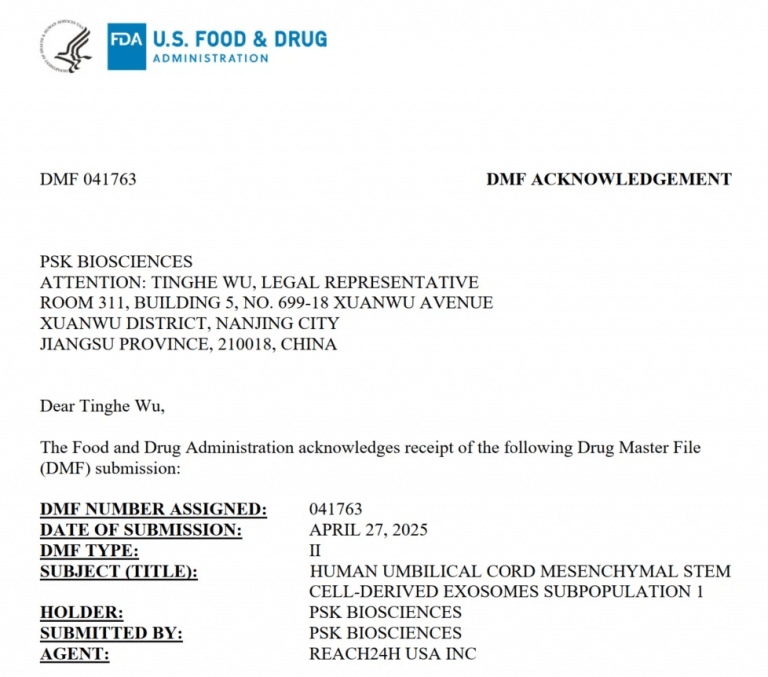
To date, Ruiou BaiPharm has supported numerous Chinese enterprises in registering their exosome products with the FDA, paving the way for entry into the international market.
Overview of the U.S. DMF Filing System
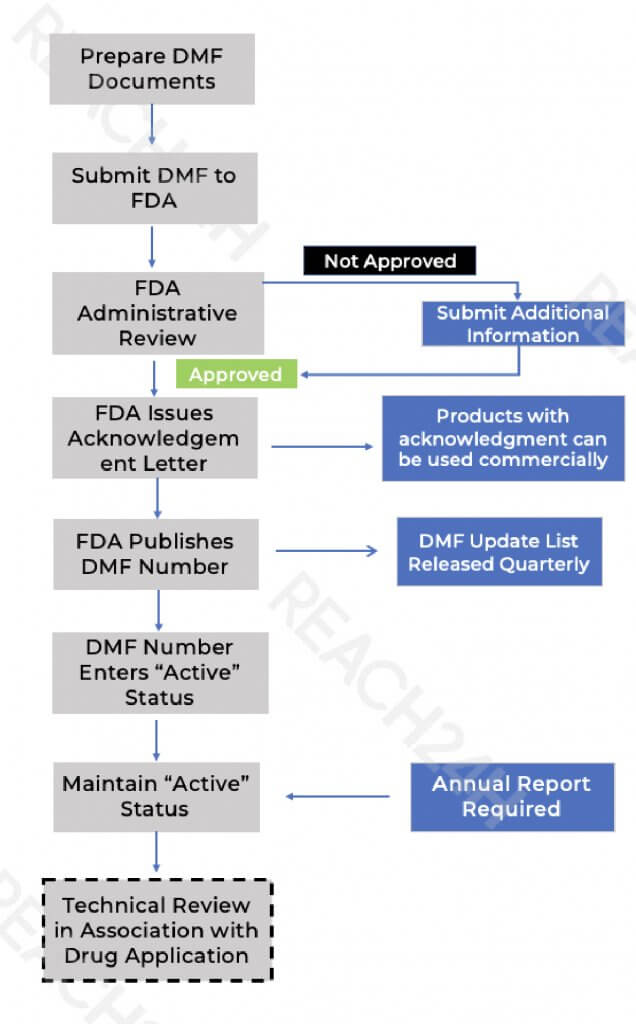
A Drug Master File (DMF) is a confidential document submitted to the U.S. FDA containing detailed information on the facilities, processes, and materials used in the manufacture, processing, packaging, and storage of drugs. While not a prerequisite for FDA approval, a DMF serves as a key reference that can support various regulatory submissions, including:
- Investigational New Drug (IND) applications
- New Drug Applications (NDA)
- Abbreviated New Drug Applications (ANDA)
- Export applications
- Amendments and supplements to the above
All DMFs are stored in the FDA’s Central Document Room (CDR) and can be cross-referenced in regulatory filings.
FDA DMF Classifications
| Type | Contents |
| Type II | Drug substances, intermediates, and drug products |
| Type III | Packaging materials |
| Type IV | Excipients, colorants, flavors, essences, and other additives |
| Type V | Clinical and non-clinical information (subject to FDA approval before submission) |
U.S. DMF Filing Process for Overseas Companies
For non-U.S.-based companies, the FDA recommends appointing a U.S. Agent to act as the official liaison. The agent’s responsibilities include:
- Acting as the primary point of contact with the FDA
- Submitting, updating, and maintaining DMF documentation
- Responding promptly to FDA inquiries or review comments
Ruiou BaiPharm’s Successful Cases
To date, Ruiou BaiPharm has completed nearly 100 successful DMF filings. Highlights include:
- Type II: Chromatography media, exosomes, stem cell lines, cryopreservation solutions, culture media, protein cofactors, active pharmaceutical ingredients, and more (30+ categories)
- Type III: HDPE barrels/bottles, cryopreservation bags/multilayer coextrusion bags, PP closure systems/aluminum-plastic composite closures, release films, pre-filled syringes/pen injectors, vials/ampoules for inhalation or injection (20+ items)
- Type IV: Pharmaceutical microcarriers, calcium alginate, gelled hydrocarbons, celluloses, polyethylene glycol, gelatin, povidone, honey, mannitol, etc. (20+ items)
Need Support with U.S. FDA DMF Filing?
Whether you are developing exosomes, stem cell therapies, or advanced biologics, REACH24H provides end-to-end solutions for successful DMF submission to the FDA. Our services include:
- Strategic regulatory planning
- DMF document preparation and submission
- U.S. agent representation
- Ongoing maintenance and FDA correspondence
Contact us today to accelerate your product’s entry into the U.S. pharmaceutical market.





